Affinity Resin Selector
Find the perfect Ecolab
resin for your monoclonal
antibody purification needs.

Find the perfect Ecolab
resin for your monoclonal
antibody purification needs.

As a global leader in resin technology, we develop and manufacture small beads that are used in the most regulated industries in the world to separate, remove or recover very specific elements and compounds.
Learn More
With 40 years of manufacturing expertise and 30 years of regulatory experience, we supply leading separation, purification and extraction technologies to support chromatography applications within the Pharma and Medical space.
Learn more
We are a world leader in resin-based separation, purification and extraction technology, that provides sustainable solutions for our environment, businesses and healthcare.
Learn More
Unsere stets einsatzbereiten technischen Support- und Serviceteams gehen noch einen Schritt weiter, um Ihre vertrauenswürdigste Ressource zu sein. Wir sind für Sie da.
Originally presented at Hydrometallurgy 2014. Reprinted with permission of the Canadian Institute of Mining, Metallurgy & Petroleum.
Johanna Van Deventer
Abstract
Volatile gold prices in the face of rising costs for mining and refining is causing the mines to look for new ways to reduce costs and increase recoveries. This task is complicated by the fact that the gold in hereto unexploited reserves is associated with increasingly complex ores — either preg-robbing, refractory, associated with high concentrations of copper, or all of the above. Ion exchange resins can play a significant role in this quest for improvement. Although ion exchange resins have been used in the Former Soviet Union (FSU) countries for the recovery of gold for many decades, it is still not widely used in the Western World. Synthetically manufactured ion exchange resins can be tailor-made to suit the chemical and mechanical characteristics of a specific application. As such, ion exchange resins can be used in a variety of solution matrices (e.g., cyanide, thiosulfate and acid), and process designs (recovery from clear liquor after solid-liquid separation or directly from the leached pulp). In this paper, a number of applications are discussed along with a review of recent developments in ion exchange technology.
Key words: Ion exchange resin, gold, preg-robbing, thiosulfate-leach, cyanide-leach, complex gold ores
Introduction
The ability of ion exchange (IX) resin manufacturers to customize key chemical and physical properties of their products makes IX resin a versatile and important technology platform for many processes and industries. A host of variables can be manipulated to match the requirements of a particular process. These include selectivity of one ion over another, raw available capacity, porosity, diffusivity, mechanical strength, mean diameter, functional groups and polymer matrix. Needless to say, a manufacturer sometimes has to choose between contradictory requirements, since promotion of a specific characteristic may result in the compromise on another.
An under-exploited application of IX resins is in the recovery of gold. Former Soviet Union (FSU) countries have always shown preferred use of ion exchange resins, especially employing resin-in pulp (RIP) technology [2]. Contrastingly, activated carbon is the preferred adsorbent for gold recovery in the Western World. Special gold-selective resins provide an economical advantage over the more commonly used activated carbon for the recovery of gold from cyanide leach-liquors. This is especially true in the case of preg-robbing ores, as well as ores containing high concentrations of undesirable base metals, especially copper.
As the "easier" ore-bodies are being exhausted, the focus is shifting to the extraction of gold from increasingly difficult and complex ores. These ore-bodies often require new processing methods, from mining through to milling, flotation, choice of lixiviant and adsorbent. The double-refractory nature of the Goldstrike ore has prompted Barrick Gold Company of Canada to develop a flowsheet using thiosulphate as an alternative lixiviant to cyanide. IX resin is the adsorbent of choice from thiosulfate leach liquors since activated carbon has no affinity for the gold-thiosulfate complex.
Theoretical Background
Chemical Characteristics of Ion Exchange Resins
Ion exchange resins typically have a styrenic or acrylic backbone, cross-linked with divinylbenzene (DVB). The structure of the resin can be either gel or macroporous. A gel resin is a clear glassy bead that is fully translucent to light. Macroporous resins contain a network of pores within a gel matrix. Light is scattered by the pores, making these resins appear opaque. Functional groups that are attached to the backbone and located throughout the resin bead interact with ions in solutions. Different functional groups can be used to ensure selectivity for specific ions. The kinetics or rate of reaction between the ions in solution and the resin is determined by the rate of ion diffusion through the thin film of water surrounding each bead (film diffusion) and through the bead itself (particle diffusion). In a dilute solution, such as during the adsorption phase, the rate of film diffusion is slower than the rate of particle diffusion. During subsequent elution, the reverse is true.
A number of special gold-selective resins are available on the market. These can be grouped roughly according to their functionality under strong-base and medium-base resins [9, 10, 12].
Strong base anion (SBA) exchange resins are more commonly used for gold extraction. These resins generally contain quaternary ammonium functional groups with fixed positive charges. Commercial examples include the Dowex-Minix and Purolite™ A193 resins. The gold loading efficiency of these resins is not pH sensitive and they operate effectively across the entire pH range. The loading of gold-cyanide onto the resin is depicted below.
P — NR3+SO4- + Au(CN)2- --> P — NR3+Au(CN)2- + 1/2SO42-
Where P represents the polymer matrix and R an alkyl chain
Due to the strong affinity of these resins for gold-cyanide anions, elution of the loaded precious metals is more difficult and a sulfuric acid/ thiourea mixture is most commonly used. During elution, the gold-cyanide complex is broken and a more stable gold-thiourea complex is formed, as shown below. The resin has no affinity for the neutral gold-thiourea complex and the gold reports to the eluate stream.
P — NR3+Au(CN)2- + 2TU + 2 H2SO4 --> P — NR3+HSO4- + Au(TU)2+HSO4- + 2HCN
A disadvantage of the use of SBA resins for gold cyanide recovery is the fact that thiourea is a suspected carcinogen, resulting in reluctance by new operations to use strong base resins. Thiourea is, however, readily oxidized and could be safely disposed of.
Medium base resins contain a mixture of quaternary, tertiary, secondary and primary amine groups. Commercial examples are Purolite S992* [currently Purogold™ MTA9920] and Henkel's Aurix (with guanidine functionality). The functional groups on medium base resins must be protonated for extraction to take place, as depicted in the equations below. Hence, these resins perform best at pH values between 9 and 11, with a reduction in gold loading capacity above pH 10.5.
Protonation: P — NR2 + H+ --> P — NR2H+
Adsorption: P — NR2H+Cl- + Au(CN)2- --> P — NR2H+Au(CN)2- + Cl-
The extent of amine protonation at a specific pH is determined by the basicity, or pKa value, of the functional group on the resin. The effect of the basicity of the functional group on loading and elution of a medium base resin is illustrated below.
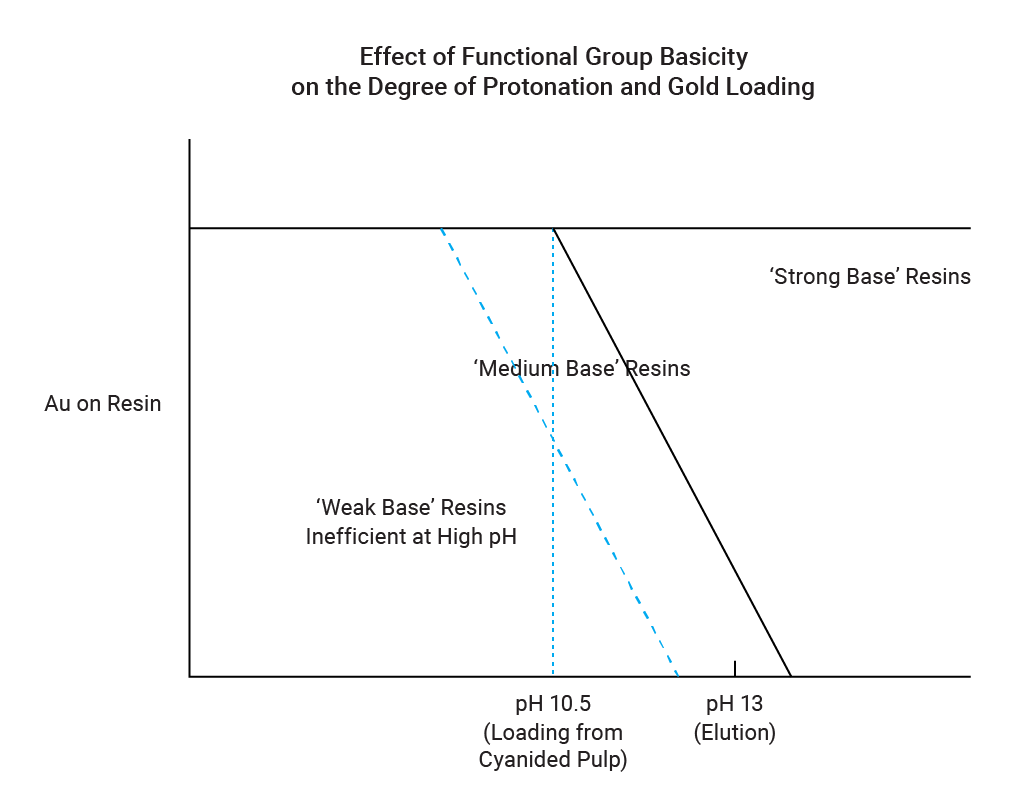
Elution of the resin is quite simple and is performed by contacting the resin with an alkali, such as sodium hydroxide. The alkali causes deprotonation of the resin, thereby removing the positive charge. The now-neutral resin has no affinity for the metal-cyanide complex and releases it. The use of an alkali for elution ensures that the pH remains alkaline throughout the adsorption and elution processes, thereby eliminating the possibility of toxic hydrogen cyanide evolution. This is a big advantage, from a health and safety point of view.
Mechanical Characteristics of Ion Exchange Resins
The most important mechanical characteristics of an adsorbent are size and strength. The diameter of ion exchange resin beads typically varies between 300 and 1200 µm with various intermediate grading sizes also available commercially. Narrow size distribution is always preferable, due to the resultant uniform reaction kinetics. The choice of bead size is dependent on the contactor design. A smaller diameter bead has a shorter diffusion path, resulting in faster kinetics. However, smaller beads cause higher pressure drops in fixed bed applications under the same conditions. Fixed-bed operations, treating clarified liquors, typically use IX resins with mean bead diameters ranging from 500 to 700 µm, with a variation of ±50 µm around the mean diameter. Resins used in RIP operation have larger beads, to ensure ease of separation of the loaded resin from the pulp. Gold ores are typically milled to 75% passing 75 µm. The apertures of the screens used to separate the resin and pulp are typically 500-600 µm. Resins used in RIP operations have a particle size ranging between 800-1200 µm, which means that the resin particles are roughly 800 µm larger than the pulp particles and roughly 200-300 µm larger than the screen apertures, allowing rapid separation. Larger beads are not practical, due to the longer diffusion path (slower kinetics) and larger surface area, which in turn, causes more rapid degradation.
Resins break down due to the combination of osmotic shock and physical abrasion. Osmotic shock is a result of swelling and shrinking of the resin when it alternates between acidic and alkaline conditions. SBA resins typically swell 5-10% from the sulfate to the hydroxide forms. In a RIP operation, the resin is intimately mixed with the pulp during adsorption. Pulps with a high clay content are relatively soft, but other pulps, such as the siliceous ores of the Witwatersrand in South Africa, are highly abrasive. Further mechanical stress occurs during screening and transfer of the resin between stages, whether it is via pumps, airlifts, or eductors. Mechanical durability of the resin is very important, especially in RIP operations.
Cyanide Leach
Solution Speciation
Cyanidation is still the most widely used method to leach gold. Either sodium or calcium cyanide can be used as lixiviant. Gold, as well as other metals present in the ore (silver, copper, cobalt, nickel, zinc, iron, etc.) forms an anionic metal-cyanide complex. This complex is adsorbed onto basic resins as well as activated carbon. The base metals can form various metal-cyanide complexes, depending on the concentration of free cyanide and the pH of the liquor [1, 15], as listed in the table below.
| Metal Cyanide Complexes Formed in Cyanide Leach Liquor | |
|---|---|
| Metal | Cyanide Complex |
| Au | [Au(CN)2]- |
| Ag | [Ag(CN)2]- |
| Cu | [Cu(CN)2]-, [Cu(CN)3]2-, [Cu(CN)4]3- |
| Co | [Co(CN)5]3-, [Co(CN)6]3- |
| Ni | [Ni(CN)]+, [Ni(CN)2], [Ni(CN)3]-, [Ni(CN)4]2- |
| Fe | [Fe(CN)6]3-, [Fe(CN)6]4- |
| Zn | [Zn(CN)4]2- |
Gold-selective ion exchange resins have been designed to have a high selectivity for the monovalent Au(CN)2- (and Ag(CN)2- ) species. Any other metals that forms similar complexes, such as Cu(CN)2-, will compete for active sites on the resin and reduce the resin's capacity for precious metals.
Ion Exchange Resins Versus Activated Carbon
While the Western World has been slow in adopting IX resins for gold recovery, several operations outside of the FSU region have successfully implemented the use of RIP/RIL in recent years. In most cases, the operation was forced to use IX resin, sometimes changing from activated carbon, due to the nature of the ore. IX resins have several advantages over activated carbon for the recovery of gold, as discussed in a number of previous publications [8, 9, 10, 11]. The main advantages are summarized below and discussed in more detail in the following paragraphs:
Improved gold recoveries from preg-robbing ores;
Better selectivity for gold over base metals, especially copper;
Simple and energy-efficient elution/regeneration
Improved Gold Recoveries from Preg-robbing Ores
Some gold ores contain naturally occurring carbonaceous material which competes with activated carbon for gold, thereby reducing the recovery efficiency. Ion exchange resins show high adsorption efficiencies, even in the presence of naturally occurring carbonaceous matter. Diesel or kerosene can be added to the pulp [11] to blind the "natural carbon," but it also fouls the activated carbon adsorbent. Resins are not fouled to the same extent as activated carbon by these additives and can be successfully used in the presence of some organic blinding agents. Avocet Mining's Penjom Gold Mine in Malaysia changed from CIL to RIL, due to the high occurrence of carbonaceous material in the ore.
Selectivity
Gold-selective resins have a higher selectivity for gold over base metals than activated carbon. This allows higher gold loadings since resin capacity is not "wasted" on undesirable base metals. A further result of the reduced affinity of resins for base metals is the fact that these metals can be preferentially eluted from the resin prior to gold and silver, thereby ensuring a cleaner eluate, higher cell-house efficiency and higher-purity bullion.
Copper proves to be the biggest problem, due to the similarity between the gold-cyanide and copper-cyanide complexes. High concentrations of copper result in high cyanide consumptions. Excess free cyanide must be maintained to minimize the formation of the Cu(CN)2- complex and avoid significant copper loading onto the activated carbon. Measures such as selective mining or removal of copper via acid leach have been tried, but are only effective to a point. Anglo Asia's Gedabek mine in Azerbaijan [13] chose the gold-selective Dowex-Minix resin over activated carbon, due to the high copper content of the ore.
Elution/ Regeneration Procedure
Gold-selective IX resins are stripped with a mixture of H2SO4/thiourea, to overcome the strong bond between the resin and gold-cyanide complex. The process is efficient and complete gold elution is achieved within 6 to 8 hours. Additional steps may be included [9] to selectively strip base metals prior to gold. Typically, a sulfuric acid strip is used to remove base metals, prior to elution of gold/silver with H2SO4/thiourea. The elution/ regeneration process of IX resins and activated carbon are compared in the table below. The process is much simpler and less energy-intensive in the case of IX resins. Resins are stripped at relatively low temperatures of around 60 °C, while activated carbon is eluted at 120-130 °C. After elution of the gold, activated carbon requires thermal regeneration at ~700 °C. Some gold-selective resins undergo a chemical regeneration with NaOH, prior to being returned to the adsorption section. No thermal regeneration is required for resins. This constitutes a significant energy saving in the case of ion exchange resins, which is especially relevant in remote areas where access to cheap and reliable power can be a problem. Diesel often has to be trucked in for the generation of power in remote locations, adding cost and logistical issues.
| Elution Characteristics of Gold-Selective Ion Exchange Resins and Activated Carbon | |||
|---|---|---|---|
| Strong Base | Activated Carbon | Comments | |
| Eluant | H2SO4/thiourea | NaOH/NaCN | Thiourea is a suspected carcinogen; evolution of toxic HCN under acidic conditions requires scrubbing. |
| Elution temperature | 55-60 °C | 110-130 °C | Carbon elution requires high temperatures; takes place in a pressure vessel. |
| Scale treatment | No additional acid wash required to remove scale | Periodic HCl wash to remove scale; HCN evolution | -- |
| Regeneration | Chemical regeneration with NaOH required for some resins | Thermal regeneration at 700-800 °C | -- |
The main disadvantage of gold-selective resins is that they are more expensive than activated carbon. However, despite the higher adsorbent cost, resins can be significantly more cost-effective than carbon, in terms of both capital and operating cost.
Copper-Gold Ores
A large portion of hitherto unexploited gold ore-bodies contain cyanide-soluble copper minerals. The presence of copper causes a number of problems, including low gold recoveries, high cyanide consumption, and consequent detoxification costs. The reason for the lower gold recoveries is that copper forms the monovalent Cu(CN)2- species, amongst others. This species competes directly with the similar looking Au(CN)2- species for capacity on both activated carbon and gold-selective IX resins. Under cyanide-deficient conditions, this is the predominant copper-cyanide species, thereby further exacerbating the situation [1, 6]. Larger adsorbent inventories are required to ensure the same gold recoveries. Some copper rejection is possible during elution, but the eluate invariably contains high copper values, resulting in reduced cell-house efficiency and bullion purity.
| Adsorbent Loading onto S992*, Activated Carbon and Commercial SBA Resin | ||||
|---|---|---|---|---|
| Metal | Adsorbent loading, mg/kg | |||
| Metal concentration in solution, mg/L | S992 [currently Purogold MTA9920*] | Commercial strong base resin | Activated carbon | |
| Au | 9 | 4183 | 9080 | 16450 |
| Cu | 13.6 | Not detected | 525 | 105 |
| Zn | 1.0 | 96 | 103 | 233 |
| Ni | 10.4 | 128 | 1746 | 129 |
* Currently Purogold MTA9920
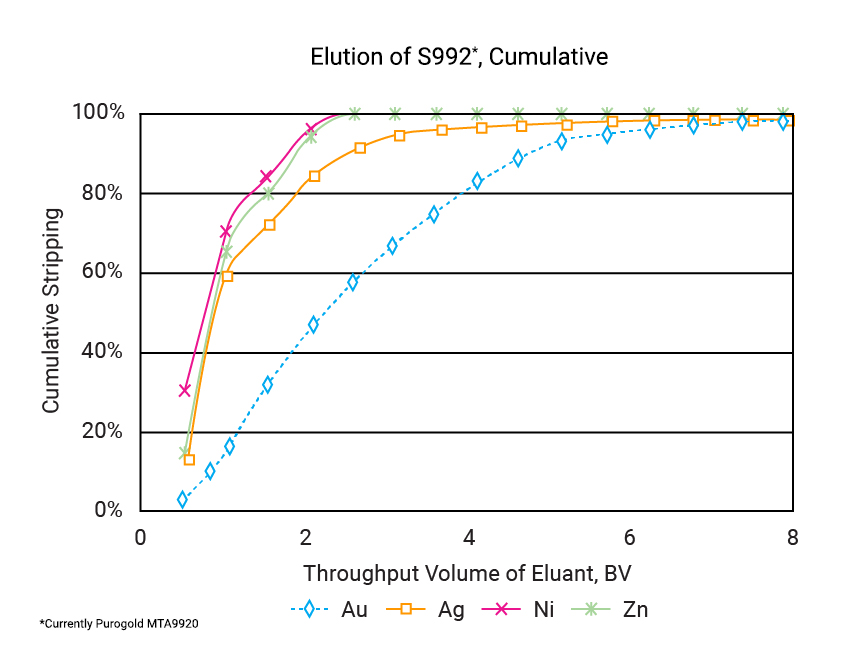
"Standard" gold-selective IX resins provide improved gold loading in the presence of copper, relative to activated carbon. Recent test work [16] showed that Purolite S992* [currently Purogold MTA9920] may be an even better option in the case of copper/ gold ores. It is a medium-base gold-selective resin, being used at Petropavlovsk's Pioneer gold mine in Russia in a RIP application [14]. Test work was done to determine the loading and elution performance of the resin, relative to a commercial gold-selective strong base resin and activated carbon. A synthetic liquor containing gold, copper, zinc and nickel was used to represent a "typical" gold-cyanide leach liquor. The free cyanide concentration was ~150 ppm and the pH of the feed was maintained at ~10.5. Metal loadings were determined by passing a large volume of solution through an adsorbent bed, in a column. This method ensured that the adsorbent was close to equilibrium with the solution. Metal loadings are listed in the table above.
The selectivity order for the three adsorbents was as follows:
S992* [currently Purogold MTA9920] medium base resin: Au > Zn > Ni >> Cu
Commercial strong base resin: Au > Ni > Zn > Cu
Activated carbon: Au > Zn > Ni > Cu
Elution was done using a mixture of 20 g/L NaCN and 10 g/L NaOH, at a temperature of 60 °C and a flowrate of 2-bed volumes per hour. Tests showed that complete elution of the resin was achieved within 8-bed volumes. The base metals were eluted before the gold and silver. The results are depicted graphically in the figure below.
This resin shows good promise for the exploitation of copper-gold ores. Since it is a medium-base resin, adsorption of gold is sensitive to pH and careful control will be required to ensure maximum loading is achieved. The gold loading was relatively low, compared to the other adsorbents. This means that larger inventories would be required. However, it may still present the only economic option for some ore-bodies. An additional advantage of this resin is the fact that it is eluted under alkaline conditions, thereby eliminating the risk of the formation of toxic hydrogen-cyanide. It also does not require energy-intensive thermal regeneration, such as activated carbon.
Further test work is underway to determine the economical limits of the resin in terms of the relative copper:gold content of the feed.
Thisulfate Leach
Thiosulfate leaching of gold (and silver) ores provides an alternative to cyanide leaching [4, 5, 7]. It provides better recoveries than cyanide in the case of preg-robbing and refractory ores. A number of systems have been investigated, characterized by the oxidant system used. These systems include copper ammonia, iron-EDTA, iron-oxalate, and oxygen. This method also shows very good promise for the treatment of gravity concentrates. Gravity concentrates are typically treated by intensive cyanidation or direct smelting. The copper-ammonia-thiosulfate system offers some unique benefits over intensive cyanidation, including:
Elimination of the use of cyanide, thus eliminating a health & environmental risk;
The dissolution of copper can be significantly lower than in a cyanide system due to lower copper mineral solubility;
The dissolution kinetics and leach recovery of silver is better than in a cyanide system.
Activated carbon has no affinity for the gold thiosulfate complex, meaning that the established processes of carbon-in-leach (CIL) and carbon-in-pulp (CIP) cannot be used for final recovery of the gold. SBA resins are used instead, in either RIP or RIL configuration.
The first commercial plant, using thiosulfate as lixiviant, is being built by Barrick Gold Corporation of Canada and is due to start-up in 2014 [5]. They have done extensive research into the use of thiosulfate as lixiviant on the Goldstrike orebody. The ore is double-refractory, i.e., the ore contains naturally occurring carbonaceous matter and the gold is occluded by sulfide minerals. Work to date included a demonstration plant that was operated for 16 months during 2010-2011. During the test program and demonstration plant, the performance of the SBA resin that will be used was scrutinized and found satisfactory in terms of gold loading, ease of elution and durability.
Low-Grade Liquors
Low-grade liquors are treated on their own, due to the exceptional sensitivity of such operations to the cost of recovering the gold. Heap-leaching provides a relatively cheap method for the recovery of gold from low-grade material. Another source of low-grade material is old mine-dumps. Historical dumps often contain an appreciable amount of gold which was not recovered due to the inefficient methods available in the past. The concentration of gold obtained from these types of operations is usually low (<1 mg/L) and the liquor may contain varying concentrations of base metals. Work was done on a milling-in-cyanide liquor, to compare activated carbon and the Dowex-Minix resin for recovery of gold from clear liquid [15].
The composition of the feed liquor used for the test work is provided in the table below. Since the feed was obtained from a milling-in-cyanide operation, the feed was particularly "difficult," as explained below:
The pH of the solution was ~7. This is below the pH at which HCN forms, contributing to the relatively low free cyanide concentration;
Low free cyanide concentration, which would favor the formation of the Cu(CN)2- complex;
High thiocyanate (SCN-) concentration, providing additional competition to the gold cyanide complex for active sites on the resin.
| Composition of Low-Grade Cyanide-Leach Feed Liquor | |
|---|---|
| Component | Concentration, mg/L |
| Au | 0.67 |
| Ag | <1 |
| Co | 7.1 |
| Cu | 9.5 |
| SCN | 184 |
| Fe | <0.5 |
| Ni | 22.6 |
| Zn | <1 |
| CN | 12.3 |
Despite these drawbacks, a preliminary economic comparison, based on the data obtained, showed that significant savings can be achieved using IX resin in a resin-in-solution (RIS) configuration. The relative estimated capital cost (CAPEX) for a RIS and carbon-in-solution (CIS) plant is compared below as well as the operating cost (OPEX). A weight of 1 has been assigned to the CIS costs, for ease of comparison. The costs were based on a feed throughput of 150 m3/h, containing 1.5 g/L gold.
| CIS vs RIS: Relative CAPEX Estimates | ||
|---|---|---|
| Resin-in-Solution | Carbon-in-Solution | |
Total CAPEX |
0.65 |
1 |
Adsorption |
1.9 |
1 |
Desorption |
0.51 |
1 |
Regeneration |
0 |
1 |
Electrowinning and Smelting |
1 |
1 |
| CIS vs RIS: Relative OPEX Estimates | ||
|---|---|---|
| Resin-in-Solution | Carbon-in-Solution | |
| Total OPEX | 0.60 | 1 |
| Adsorbent | 0.44 | 1 |
| Reagents | 0.55 | 1 |
| Power/Diesel | 0.43 | 1 |
| Labor | 1 | 1 |
| Maintenance | 0.57 | 1 |
The economic comparison indicated that IX resins provide a cost-efficient alternative to activated carbon, with a CAPEX saving of ~35% and OPEX saving of ~40 % in the case of a RIS plant when compared to CIS.
Future Resin Developments
Another new development is the production of IX resins with an inert core [2]. The functional groups of the resin are contained in the outer portion of the bead, only. We call these Shallow Shell™ resin. The advantages include faster kinetics, more uniform reaction kinetics and a reduction in reagent consumption.
For an exchange of ions to take place between the solution being treated and the functional groups inside the resin, the solution has to diffuse into the resin bead. Smaller diameter IX beads, also referred to as 'fine mesh' resins, exhibit faster kinetics than larger beads, due to the shorter diffusion path and increased surface area. The drawback of fine mesh resins is the higher pressure drop experienced across the resin bed in the case of fixed-bed operations. Shallow Shell resins are manufactured with a functionalized outer shell and an inert core. Therefore, all of the ion exchange takes place in the functionalized shell of the resin. The figure below shows how a Shallow Shell resin bead with an average diameter of 0.625 mm has the effective diffusion path length of a fine mesh resin bead with an equivalent diameter of 0.375 mm. Since the rate of diffusion within the resin bead is inversely proportional to the square of the radius of the bead (1/r2), the rate of diffusion within a resin bead with a diameter of 0.37 mm is therefore 2.78 times that in a bead with a diameter of 0.625 mm. The Shallow Shell resin matches the loading and regeneration efficiency of a fine mesh resin but uses a large diameter bead that does not have the higher pressure drop limitations associated with fine mesh resin.
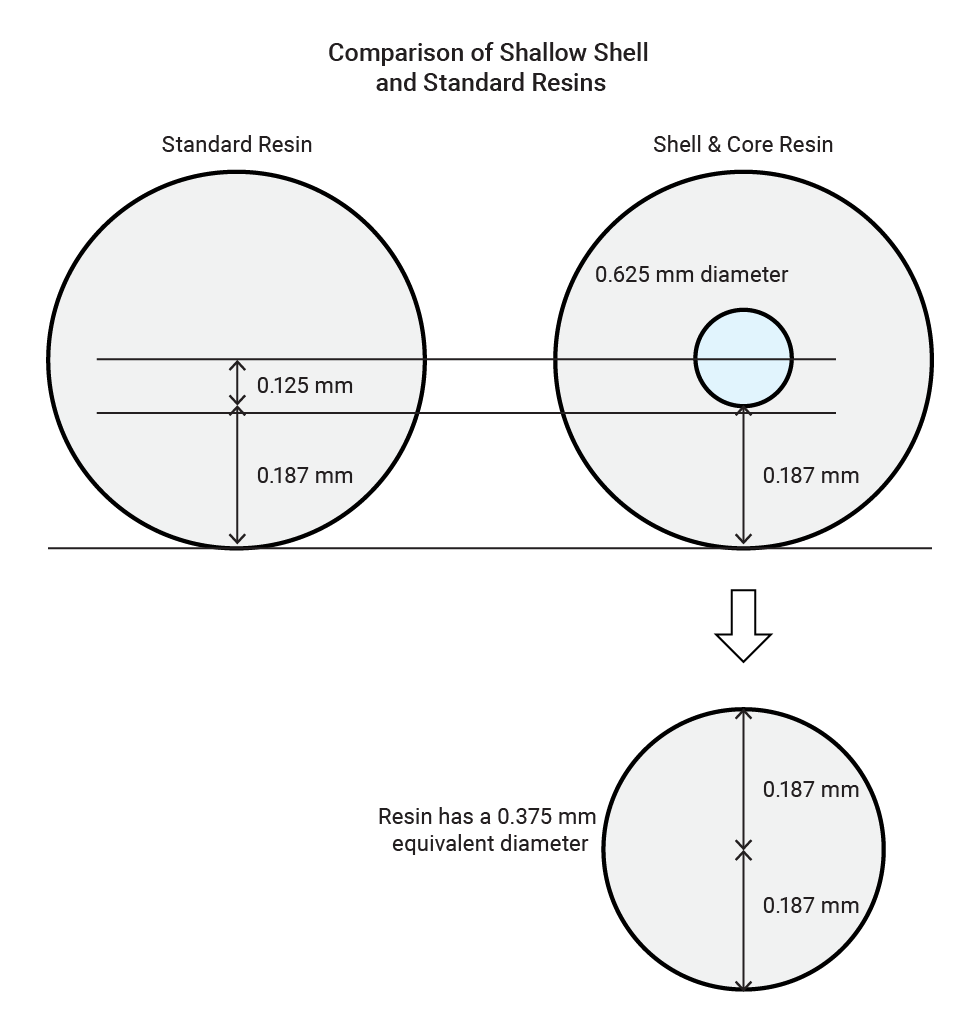
This technology was initially developed for the industrial water treatment industry but will find application in the metallurgical industry in any application that recovers metals from clear liquid in a fixed bed configuration. The development of shallow shell resins containing functional groups aimed at specific processes, apart from water treatment, are at an advanced stage.
Conclusions
Ion exchange resins are expected to play an ever-increasing role in improving recoveries and reducing costs and environmental risks for gold mining operations. Gold-selective resins have potentially higher gold loadings and improved selectivity than activated carbon, thereby ensuring higher-purity bullion. Probably the biggest advantage of resins over carbon is their reduced energy requirement. Activated carbon is eluted at elevated temperatures of 110-130 °C, while resins are eluted at 55-60 °C. In addition, carbon requires thermal regeneration at 700-800 °C, while resins require only chemical regeneration. This is particularly important for mines in remote locations. Reliable and cheap power is often not readily available and the mines are reliant on diesel that has to be trucked in, adding cost and logistical problems.
Medium-base resins provide a further health & safety advantage, since these resins are eluted under alkaline conditions, thereby eliminating the risk of toxic hydrogen cyanide evolution.
A recent development in the gold-recovery flowsheet is the use of thiosulphate as lixiviant as alternative to cyanide. Some ore-bodies are considered double-refractory, where the gold is occluded by sulfide-minerals and the ore contains native carbon that acts as a preg-robber. In such cases, thiosulphate provides the only viable option. Thiosulphate is also considered as less environmentally hazardous than cyanide. However, activated carbon cannot be used as adsorbent, since it has no affinity for the gold thiosulphate complex. Strong base ion exchange resins are used as adsorbent instead.
References
Adams, M., Lawrence, R., Bratty, M. (2008). Biogenic sulfide for cyanide recycle and copper recovery in gold-copper ore processing. Minerals Engineering 21 pp. 509-517. Elsevier Ltd.
Bolinsky, L., Shirley, J. (1996). Russian resin-in-pulp technology, current status and recent developments. Randol Gold Forum '96, pp 419-423. Golden, CO: Randol International.
Boodoo, F., Rosie, J. (2010). Ion exchange synergy with other water treatment technologies: The way of the future. Caloundra, Australia: Australian Power Institute.
Breuer, P., Dai, X., Zhang, H., Hewitt, D. (2012). The increased activity in the development of thiosulphate based processes for gold recovery. In ALTA 2012 Gold Conference. Melbourne, Australia: ALTA Metallurgical Services.
Choi, Y. (2013). Thiosulphate processing: from lab curiosity to commercial application. In ALTA 2013 Gold Conference. Melbourne, Australia: ALTA Metallurgical Services.
Dai, X., Simons, A., Breuer, P. (2011). A review of copper cyanide recovery technologies for the cyanidation of copper containing gold ores. Minerals Engineering 25, pp 1-13. Elsevier Ltd.
Fleming, C.A., McMullen, J., Thomas, K.G., Wells, J.A. (2003). Recent advances in the development of an alternative to the cyanidation process: thiosulphate leaching and resin in pulp. Englewood, CO: Society for Mining, Metallurgy, and Explorations, Inc.
Green, B.R., Kotze, M.H., Wyethe, J.P. (2002). Developments in Ion Exchange: The Mintek Perspective. JOM, Volume 54, Issue 10, pp37-43. Warrendale, PA: The Minerals, Metals and Materials Society.
Kotze, M. (2010). Gold Ion Exchange. In ALTA 2010 Gold Conference. Melbourne, Australia: ALTA Metallurgical Services.
Kotze, M., Green, B., Mackenzie, M., Virnig, M. (2005). Resin-in-pulp and resin-in-solution. In Volume 15: Advances in Gold Ore Processing. Editor: M. Adams, Elsevier Science.
Lewis, G.V. (2000). The Penjom Process: An Innovative Approach to Extracting Gold from Carbonaceous Ores. In Gold Processing in the 21st Century: An International Forum. Perth, Australia: A.J. Parker Cooperative Research Centre for Hydrometallurgy.
Marston, C.R., Gisch, D.J. (2010). New Selective Strong Base Anion Exchange Resins with promise for Commercial Gold Cyanidation. In ALTA 2010 Gold Conference. Melbourne, Australia: ALTA Metallurgical Services.
Mintek-designed resin for Azerbaijan gold project. In Mintek 75 Bulletin, Issue No. 148, September 2009.
Petropavlovsk website. www. petropavlovs k.net/en/pioneer /pioneer-technology.html.
Van Deventer, J., Wyethe, J.P., Kotze, M.H, Shannon, J. (1999). Comparison of resin-in-solution and carbon-in-solution for the recovery of gold from clarified solutions. In Extraction Metallurgy 1999. Johannesburg, South Africa: South African Institute of Mining and Metallurgy.
Van Deventer, J., Kotze, M., Yahorava, V. (2012). Gold recovery from copper-rich ores employing the Purolite S992* [currently Purogold MTA9920] gold-selective ion exchange resin. In ALTA 2012 Gold Conference. Melbourne, Australia: ALTA Metallurgical Services.
Editor's Note
* Since the publication of this white paper, this product name has changed from S992 to Purogold MTA9920.
Purolite.com uses cookies to give you the best possible experience. By using Purolite.com, you consent to our use of cookies. If you do not wish to receive our cookies, adjust your browser settings. Read our Cookies Policy to learn more.